In-Situ Molecular Manipulation
Introduction
Energy sources are vital to sustain and grow the world economy. As of today, the world mainly relies on fossil fuel as the source of energy for transportation, power generation, chemicals manufacturing, and other industrial applications. The conventional sources of hydrocarbon are steadily declining; however, the oil and gas industry has been successful in finding unconventional hydrocarbons, such as heavy oil and shale gas. There are distinct challenges in producing and processing the hydrocarbons from unconventional sources into usable end products. Reducing the footprint during the production of oil, refined products, and gas will benefit the industry by reducing the overall cost and improving the health, safety, and environmental impact.
Another source of energy is renewable sources, such as sun, wind, geothermal, biomass, plant seeds, and algae. Producing usable energy from these sources and making it available to the end user pose unique challenges and opportunities. Research to understand the molecular building blocks of organisms living in diverse sources could help optimize the production of usable energy from both fossil and renewable sources. The search for microorganisms should include diverse sources, ranging from hydrocarbon reservoir to the guts of insects such as termites. Research into the molecular structure of these organisms could pave the way for improving exploration, production, and processing of fossil fuels and also help to produce usable energy from renewable sources efficiently and cost-effectively.
A game-changing technology that could benefit the industry is the in-situ molecular manipulation of the contents of challenging reservoirs. The approach is to modify the contents of the reservoir at its source so that their harsh effects are reduced or eliminated and the reservoir can be produced efficiently. For example, converting heavy oil at its source to lighter grade oil will reduce or eliminate the need for thermal recovery, the associated capital investment, and the associated environmental issues. Refining operations will be more predictable, less costly, and have a smaller carbon footprint. Converting sour gas to sweet gas will reduce or eliminate the associated process equipment at the wellsite, and health, safety, and corrosion issues. The CO2 may be manipulated to make it helpful in the reservoir, or at least make it less harmful.
Biodegradable materials could be a significant source of energy in the coming years. Gamechanging technologies that produce (1) higher-oil-yielding biodegradable materials and (2) microorganisms that efficiently convert biodegradable materials to biofuel could greatly benefit the industry. Research into increasing the amount of oil in an energy crop, such as algae, Jatropha plant seeds, and grass, could improve the oil yield measured in gal/acre. Improving the drought tolerance of an energy crop, such as the Jatropha plant, could make it attractive to cultivate because it does not compete with food crops for water and land.
The challenges before us to meet the energy demand in a safe, clean, and cost-effective manner are real. It is possible to meet these challenges and deliver safe and clean energy in a cost-effective manner by working across different disciplines of engineering, science, and business.
Opportunity
The upstream and downstream sides of the energy sector, like most other industries, face challenges in the areas of exploration, production, and refining. These also pose unique opportunities to re-evaluate the processes and develop game-changing technologies to improve the overall efficiency. The game-changing technologies should improve the economics of the process and also help to improve safety and reliability of the activity.
For example, if hazardous materials such as CO2 and H2S, are treated downhole and not brought to the surface, then this not only improves the health and safety, but also reduces the operating cost by eliminating the need to treat and dispose these materials. Needless to say, the process should be optimized so that the incremental savings are greater than the incremental cost.
Between 1978 and 1996 the US Department of Energy explored the potential of using algae to produce fuel. This program was abandoned when the crude oil price dropped from USD 50/bbl to USD 20/bbl. The situation is much different today, however, with the crude oil price around USD 100/bbl in 2011, after reaching about USD 150/bbl in 2008. The world is a much different place today than it was in 1978. The demand for energy is increasing steadily, and most of it is coming from China and India, which are growing at near double digits every year. This fuels the energy demand for transportation, power generation, chemicals manufacturing, and other industrial applications.
The increasing demand for energy is expected to provide the much-needed financial incentive to work on both improving the exploration, production, and refining of fossil fuels and redoubling the efforts to effectively use renewable sources for energy. The incremental barrel of hydrocarbon is getting heavier, and many also believe that the incremental conventional cubic foot of natural gas is getting sourer. This will place an ever-increasing burden on processing equipment, as discussed below. This represents an excellent opportunity to virtually transform the current paradigm of production and refining. In-situ molecular manipulation offers the promise of producing fluids requiring minimal treatment. This game-changing technology can be applied to deriving usable end products from both fossil and biodegradable source of energy. The successful application of the technology could blur the distinction between upstream and downstream operations.
Heavy Oil
Heavy oil is abundant in different parts of the world, such as the western part of the US, Canada, Venezuela, Brazil, Congo, and Indonesia. However, the oil is very viscous and hence can’t be produced by conventional methods. In a number of cases, the heavy oil is thermally treated to reduce the viscosity and thus make it easier to flow. In the majority of the cases, steam is generated on the surface and then injected into the reservoir to heat the heavy oil and reduce its viscosity, as Fig. 1 illustrates. Another shortcoming is that, even after successful production, the viscosity is usually too great for pipeline transportation. Expensive diluents are used to reduce viscosity to enable flow.
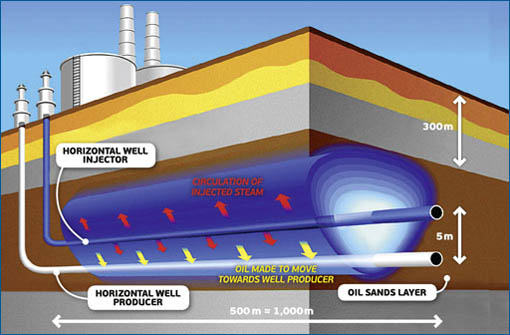
Fig. 1 - A typical steam-assisted gravity drainage process for producing heavy oil.
Enhanced Recovery Methods for Heavy Oil Bitumen and Its limitations. Existing Recovery technologies are grossly inefficient; in particular, thermal processes for heavy oil bitumen (HOB) production. In the typical steam-assisted gravity drainage (SAGD) or cyclic steam stimulation (CSS) processes, substantial volumes of water in the form of steam are used to convey thermal energy to temporarily reduce bitumen viscosity. At the surface, bitumen is subsequently cooled. While energy conservation is employed, thermal losses remain significant. HOB is once again temporarily softened (this time by the addition of a diluents) for transportation to either upgraders or remote refineries, where the diluent is stripped and transported back, increasing costs and environmental impact. During local upgrading, thermal energy is once more added to bitumen to now effect a permanent viscosity reduction by means of coking, thermal cracking, and hydrogenation reactions facilitated by catalysts to clean and stabilize the products. A substantial amount of thermal energy during steam injection could be lost to the surroundings as the steam flows from the surface to the reservoir. For instance, the quality of the steam could decrease from 80% at the surface to 30% when it is injected into the reservoir. This process is obviously inefficient and opportunity exists to improve the efficiency and reduce the footprint.
For example, the thermal energy can be generated downhole, near the reservoir and heat losses can be reduced substantially. A miniature steam-generating device can be designed and installed downhole close to the reservoir, or heating elements can be strategically wrapped around the casing close to the reservoir. One such device using efficient combustion to generate steam in-situ is currently in field trials.
Another option is to inject selected chemical components into the reservoir that will undergo exothermic chemical reaction when initiated and thus reduce the viscosity of the heavy oil. This may need steam to be injected to initiate the reaction, but then the reaction will propagate and release the thermal energy. The chemical components need to be selected for their size, shape, and reactivity so that they can be injected into the reservoir suitably. The developments in nano material technology should help the industry in achieving this goal.
The in-situ manipulation of heavy oil can be taken a step further where the heavy oil is catalytically treated to change the molecular structure so that the heavy oil is transformed into lighter fractions. In the conventional process, the heavy oil would have been brought to the surface and treated in specially built refineries (upgraders) to crack the heavy oil. By catalytically treating the heavy oil downhole, the lighter fraction can be easily handled for further processing in refineries that do not need special processing.
Treating the heavy oil in-situ and manipulating the molecules is truly a game-changing technology. Using technically advanced products and processes, HOB is converted to end products in the reservoir itself, and the polluting gases, such as CO2, are not brought to the surface by sequestration and/or adsorption. This should improve the economics of the whole upstream and downstream of the heavy oil business. A holistic approach should be taken to evaluate the economics and health, safety and environment benefits of this game changing technology
Kinetics and Catalysis and Transport. Hybrid processes have significant potential for in-situ upgrading. The catalysts for this must be transportable within the reservoir media. Ultradispersed (UD) catalyst development, manufacture, and process demonstration for in-situ application will generate substantially higher-quality petroleum in-situ with reduced needs for energy introduction to the reservoir. There is a wide area available for innovation in the formulation of UD catalysts for in-situ upgrading. The problem of catalyst access to the big bitumen molecules and their molecular aggregates can also be solved by using smaller (nano-size) catalyst particles. The incorporation of UD catalysts directly in the reservoir is feasible, as many have shown (Behdad, Kantzas, Pereira-Almao, and Larter 2010).
In-situ catalytic upgrading requires providing a means of intimate contact between catalyst, reactants, and HOB in the reservoir. This can be achieved only by placing the catalyst in an appropriate segment of the reservoir through which oil at the required temperature can be made to flow and mix with the hydrogen. Such controlled placement requires propagation of the catalyst in the reservoir over distances in the order of 1 to 10 m.
Conventional hydrocracking catalysts are large, millimeter-size, solid particles used in packed bed reactors. These catalysts cannot be dispersed in the reservoir porous media. However, UD catalysts are small enough to flow through and be dispersed within the reservoir. If the catalyst carrier is oil, the catalysts could flow with injected oil. It is also possible to carry the catalyst, or catalyst precursors, in water. Vapor is not a viable carrier for these submicron particles because the low density and high velocity of the vapor mean that the particles are more readily intercepted by the sand and deposited prematurely.
Flow of UD Particles. Little is known about the flow of UD particles in reservoir porous media. The scientific literature on the propagation of nano-dispersed particles is very scant, especially in nonaqueous media. In particular, the impact of heat transfer in determining the efficiency of transport and the displacement of fluids in porous media is essential for predicting the displacement within the reservoir and to guide choices of injection parameters. Recently, the dynamic of nano particles in pipelines and in porous media has acquired more relevance. Assessments on navigability, retention, effect of temperature, and viscosity on the agglomeration of suspended nanoparticles have been obtained for some few specific solids. It is expected that part of the injected catalyst will be retained by formation sand through a deep-bed-filtration process and that this effect will vary with flow conditions, such as sand permeability, flow velocity, UD-catalyst-particle size distribution, temperature, and presence or absence of fluids such as gas, connate water, and steam.
Modeling and Simulation. As game-changing technologies for in-situ upgrading are being developed, the laboratory tests must be simulated so that further understanding and evaluation of the underlying physics is possible (Galarraga and Pereira-Almao 2010). This means that experimental results not only need to be analyzed, but a tuned flow/transport/kinetic model needs to be constructed that contains all relevant physics. The modeling and simulation work integrates theory, experiments, modeling, and field responses for these processes. A process based on UD catalysts needs to be fully described, and as research proceeds and develops new information, it is likely that an optimum process will evolve. The process needs to be defined to the extent that an initial numerical model can be built. Results can then be used to design physical modeling experiments and to identify targets for experimentation.
Microorganisms Approach to Convert Heavy Oil to Light Fractions. The presence of heavy oil is attributed to biodegradation of crude oil via methanogenesis in subsurface petroleum reservoirs (Roadifer 1987). Methanogenesis1 or biomethanation is the formation of methane by microbes known as methanogens. Production of methane is an important and widespread form of microbial metabolism. In most environments, it is the final step in decomposition of biomass.
Higher levels of biodegraded or heavy oil are found closer to the surface. This is attributed to the aerobic bacterial degradation of shallow subsurface petroleum reservoirs. However, recent study suggests that anaerobic degradation processes dominate subsurface degradation of the crude oil despite slow reaction kinetics (Jones et al. 2008).
Researchers have discovered a possible new species of bacteria that survives by producing and “breathing” its own oxygen. The finding suggests (Mascarelli 2010) that some microbes could have thrived without oxygen-producing plants on the early Earth (and on other planets) by using their own oxygen to garner energy from methane (CH4).
"The mechanism we have now discovered shows that, long ago, these organisms could have exploited the methane sources on Earth and possibly on other planets and moons by mechanisms that we didn't know existed," says Mike Jetten, a microbiologist (Jones et al. 2008) at Radboud University Nijmegen in the Netherlands and part of the team that conducted the study. The oxygen-producing bacterium, provisionally named Methylomirabilis oxyfera, grows in a layer of methane-rich but oxygen-poor mud at the bottom of rivers and lakes. The microbes live on a diet of methane and nitrogen oxides, such as nitrite and nitrate. These nitrogen-containing compounds are especially abundant in sediment contaminated by agricultural runoff (Ettwig 2010).
A number of researchers are searching for organisms that could convert heavy oil to a lighter version or quality. Until only a few years ago, the majority of researchers were of the opinion that no living matter could exist in hydrocarbon reservoirs. This is despite the discovery of hyperthermophilic life in Yellowstone geysers as early as the 1960s. Thermophiles are organisms that thrive in hot temperatures, between 45°C and 80° C°2. The hyperthermophiles are extreme thermophiles for which the optimum temperatures are above 80°C°. Hans Kristian Kotlar (2009) reports that Statoil has gathered a number of organisms with an exceptional bioconverting activity for heavy oil. Under experimental conditions, they have seen complete conversion of heavy oil to lower oil viscosities within 2 to 3 days of the addition of certain bacterial strains. These organisms are currently being analyzed at the genetic level.
These bacterial strains have not been tested in the field. A basic understanding of the molecular building blocks of the bacterial strain and results from field testing could help in optimizing the process. The identification of pathways inherent in subsurface biodegradation facilitates the engineering of process to accelerate naturally slow methanogenic biodegradation to recover energy from heavy oil reservoir as methane. The process of testing and proving the concept can be accelerated by creating a team of engineers and scientists to understand the basic fundamentals and then scale-up for field testing and optimization.
The most degradable compounds in crude oil are straight n-alkanes, followed by moreresistant branch acyclic and monocyclic hydrocarbons. Jones and coworkers from different continents and different academic disciplines (Jones et al. 2008) have compared their laboratory results with the gas and oil samples taken at wellheads. These and other authors (Krejci-Graf 1932, Palmer 1993) conclude that the occurrence of anaerobic degradation of crude oils in subsurface reservoirs under methanogenic conditions explains the consistent hydrocarbon compositional pattern seen in degraded oils worldwide and their association with dry gas accumulations.
Fig. 2 shows (Jones et al. 2008) the ratio of pristane to n-heptadecane against 4-methyl biphenyl to 3-methyl biphenyl. Pristane (2, 6, 10, 14-tetramethylpentadecane) is relatively inert and thus is useful as a biological marker. Jones et al. (2008) exposed nondegraded oil from a North Sea field to laboratory microcosms and measured various parameters. Fig. 2 shows that after 686 days of subjecting the nondegraded crude oil to microcosm in the laboratory, nC17 and other alkanes are completely removed. The laboratory methanogenic microcosm data and the field data plot along the same trajectory with n-alkane degradation but no apparent aromatic hydrocarbon degradation. Fig. 3 plots the methane generated and nC7 to nC34 alkanes as a function of time when nondegraded crude oil is subjected to methanogenic conditions in the laboratory. This figure clearly shows the depletion of alaknes and the generation of methane.
The authors discuss the pathway for bacteria attack on crude oil. The first step is break down of the long-chain hydrocarbons into acetic acid. They then outline two possible pathways for formation of methane. One is conversion of acetic acid to methane and the second is oxidation of acetic acid to carbon dioxide and hydrogen and subsequently to methane. They have compared the carbon isotopic composition of carbon dioxide and methane from gases co-produced with highly degraded oils from the Peace River Oil Sands area of Western Canada.
Fig. 4 shows (Mascarelli 2010) a plot of the measured and modeled methane and carbon dioxide stable carbon isotopic compositions from degraded oil reservoir gases. The authors conclude that the highly enriched CO2 carbon isotopic signatures in heavily degraded oils and laboratory data both suggest that methanogenic hydrocarbon degradation occurs predominantly through oxidation of alkanes, followed by oxidation of acetic acid to carbon dioxide and water and then to methane. There is a suggestion (Sanderson 2007) for adding external CO2 and bacteria to a large depleted oil reservoir to produce methane. The reasoning is that bacteria would help make methane from heavy oil and produce natural gas. This is put forward as an interesting new aspect of geological sequestration of CO2.
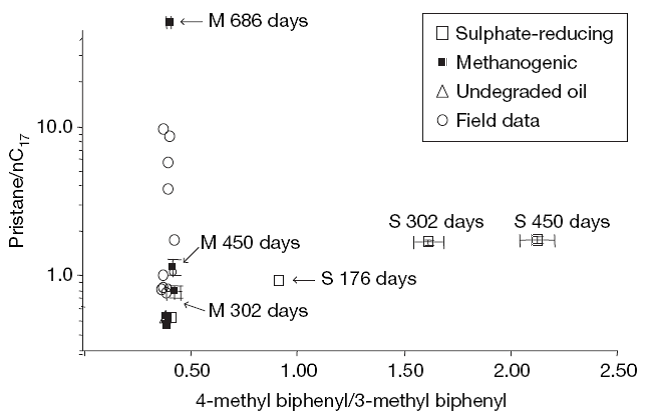
Fig. 2 - Plot of the ratios of pristane to n-heptadecane against 4-methyl biphenyl to 3-methyl biphenyl. (Nature, Vol. 451, January 2008)
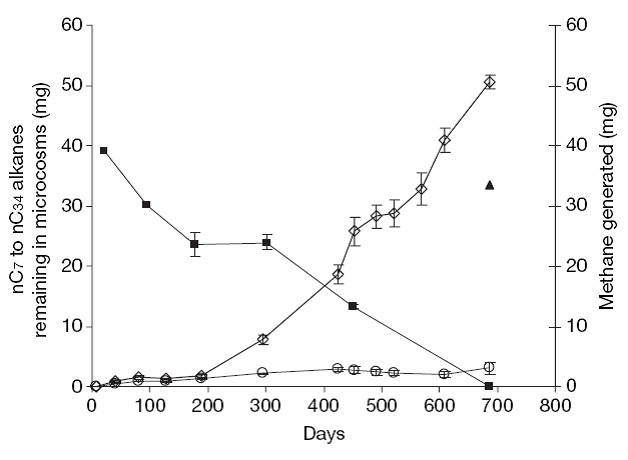
Fig. 3 - Plot of the n-alkane depletion and methane production during oil degradation. (Nature, Vol. 451, January 2008)
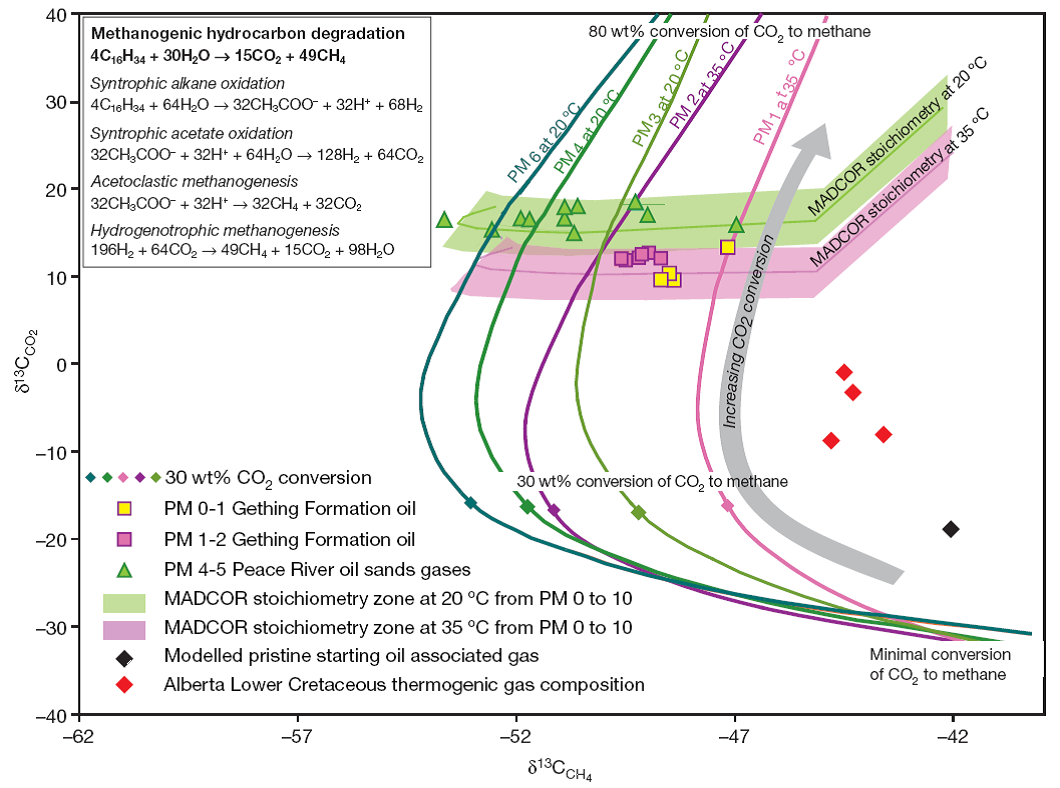
Fig. 4 - Measured and modeled methane and carbon dioxide stable carbon isotopic compositions
from degraded oil reservoir gases. (Nature, Vol. 451, January 2008)
Alternatives to Fossil Fuel
The projected demand for energy is persuasive, mainly from the growing economies of the world such as India and China. Fossil fuel by itself, whether it is from conventional or unconventional sources, may not be sufficient to meet the demands in the coming decades. This is an excellent time to investigate the feasibility of producing commercial quantity of energy from other sources such as biomass and solar. A short review of biofuel is give below.
Biofuel
The sources of biofuels are varied and include both food and non-food or energy crops. The focus is to move away from food crops, such as corn and sugarcane, to prevent skyrocketing food prices and food shortages, as observed during 2007-2008. A number of private companies and venture capitalists have invested money into developing energy crops, such as algae, Jatropha plant seeds, and high-oil-yielding grass. Recently, there has been significant money invested in algae development for oil. The advantages of algae is that they do not take away precious land that is usually needed to grow food crops and oil produced per acre may be higher than the other sources.
The basic concept (Grant 2009) behind algal biofuels is deceptively simple. Microalgae naturally produce and store lipids similar to those found in most vegetable oils. If scientists can genetically jigger the oil-storing tendencies of algae into becoming more efficient than they are in nature, commercially viable levels of transportation fuels may result. The key challenges include selecting the most suitable algae strains, growing these algal cells at optimal rates, engineering the metabolic pathways that control oil production to create cells pregnant with desirable oil products, and extracting the oil in an efficient and economic manner.
No longer lowly pond scum, algae have rocketed in status to what some say is the most promising “green” fuel source of the future, with the likes of Bill Gates, the US military, and ExxonMobil trumpeting their potential. "It's hard to find someone on the sidewalk in New York who hasn't heard about the idea of using algae for energy," says Harrison Dillon, president and chief technology officer of Solazyme, a biotechnology company in South San Francisco, California. The company signed an USD 8.5-million deal to produce commercial quantities of algal fuel for the US Navy (Mascarelli 2009).
Key players in the algal fuel race include Solix Biofuels, a Colorado-based operation that plans on firing up a closed-tank bioreactor system that uses waste carbon dioxide from beer making, and Aquaflow Binomics, a New Zealand company that seeks to produce biofuels by harvesting wild algae from polluted waterways. The first algae-powered commercial aircraft test flight, a Continental Airlines Boeing 737 was powered in part by an algal biofuel produced by California-based Sapphire Energy (de Ferra 2007).
Christoph Benning, a plant biochemist at Michigan State University admits that biologists lack a full understanding of the metabolic pathways algae use to produce oil. "We're missing the basic tools," he says.
Juergen Polle at Brooklyn College is looking for efficient oil producers, algae that can accumulate anything more than 30% of their body weight in oils. Walter Kozumbo, manager of the Air Force Office of Scientific Research's bioenergy says the triacylglycerides that photosynthetic algae accumulate generally resemble JP8, the kerosene-based jet fuel of choice for military aircraft. And these unicellular plants don't just make and store these useful oils; they can really crank them out. The US DOE says that microalgae have the potential to produce 100 times more oil per acre than any terrestrial plants, including soybeans (Grant 2009).
Algae's photosynthetic cells produce oily goo, including various oils and ethanol, that can be converted into advanced biofuels. Since 2007, more than USD 1 billion has been injected into algae-to-energy research and development, says Will Thurmond, president of Emerging Markets Online, an energy consulting firm in Houston, Texas.
Algae have several key traits that make them a desirable energy source. They can be grown on nonagricultural land in a fraction of the area required by conventional oil crops like maize (corn), soybeans, and palm. In addition, algae capture carbon dioxide and can thrive in domestic waste water or salt water. But experts warn that there are still high hurdles to overcome before algal biofuels can compete economically with conventional fossil fuels. Challenges include finding strains of algae that reliably produce high yields, keeping contamination at bay, developing cost-effective growth chambers and efficiently harvesting oil from the cells. "In the end, it's all going to come down to economics and what it's going to cost to produce this algal oil on a large, commercial scale on a dollar-per-gallon basis," says Al Darzins, who leads the algal biofuels program at the National Renewable Energy Laboratory (NREL) in Golden, Colorado.
To date, the biggest investment boon for algae has come from oil giant ExxonMobil, which announced that it will invest USD 600 million over 5 to 6 years in a partnership with Synthetic Genomics, a company in La Jolla, California, co-founded by genomics pioneer J. Craig Venter. ExxonMobil has said that its investment is contingent on Synthetic Genomics achieving certain milestones, and that if their efforts are successful, it expects to spend billions more on final development and early commercialization of algal biofuels. Synthetic Genomics is in the early stages of its venture, but the company maintains that it has engineered algal cells that can directly secrete hydrocarbons in pure form, in contrast to the standard process of splitting the cells open to harvest their oils.
Other major oil players that have entered the algal arena include Chevron, which partnered the NREL (National Renewable Energy Laboratory) in 2007 and helped to restart, with an undisclosed amount, a research program that had been shuttered since 1996. Chevron also announced a deal with Solazyme in January 2008, although the partners will offer only vague details about the nature of their collaboration.
In addition, Dow Chemical Company is backing Algenol Biofuels, a company in Bonita Springs, Florida, that specializes in ethanol production from algae, in its quest for a USD 25- million grant from the US DOE. If Algenol gets the grant, it will construct a pilot plant with Dow at Dow's manufacturing site in Freeport, Texas, with the goal of capturing industrial carbon dioxide and producing alga-derived ethanol to generate ethylene, a building block for plastics.
Meanwhile, Sapphire Energy has garnered more than USD 100 million from bigwig investors, including Gates's Cascade Investments and the Rockefeller family's venture-capital firm Venrock. Sapphire is using genetic engineering to boost several algal traits, including improved protection from predators and low-cost harvestability. It is also working to genetically manipulate algae to produce oils that are nearly identical to crude oil extracted from the ground.
One of the biggest challenges is to reproduce laboratory conditions on a large scale. In the laboratory, it can be easier to control algal growth and to find strains that produce copious amounts of oil. "But it's a totally different story when you take this organism that behaves well in the laboratory and you put it in acres' worth of outdoor ponds," says Darzins. For this reason, some companies have opted to grow their algae in enclosed “bioreactors.” But the costs of building bioreactors can be prohibitively expensive. The algae community is "still torn" between open ponds and closed bioreactors, Darzins says.
One Israeli company, Seambiotic, maintains a 1,000-m2 site with eight oblong ponds that can produce approximately 23 g/m2/day of algae, according to its scientific advisor and algal growth expert Ami Ben-Amotz. That growth rate approaches US DOE's stated (but never reached) goal of 50 g/m2/day. With the help of seawater and free carbon dioxide from Israeli Electric Company smokestacks, Ben-Amotz says that Seambiotic's only limitation to increasing that growth rate is developing a better hydrodynamic system to churn the pond water more efficiently for proper aeration and increased algal growth rates. He's working with NASA on that one. "They got to the moon," Ben-Amotz says. "I hope they will solve the problem of water mixing!" Ben-Amotz says he thinks he can eventually achieve a growth rate of about 75 g/m2/day.
Next year Ben-Amotz says that Seambiotic expects to open a new open-pond facility (again sited at an electric plant) that will likely be the largest facility for algae production in the world. It will cover 5 hectares and will provide tons of algae to different production facilities; lipids will go to biodiesel manufacturers, sugars will go to bioethanol producers, and proteins to makers of nutraceuticals. But even Ben-Amotz admits that Israel doesn't have enough land to support truly commercial-scale algae production. He says that similar facilities will need to be constructed in other countries in South and North America for that to become reality.
The challenges remain both in understanding the basic fundamentals and the pathways algae use to produce oil and also in the scale-up to produce commercial quantity oil from algae. This again needs an interdisciplinary team of experts from both engineering and basic science to understand the issues and find solutions to the forthcoming energy challenges.
Other Applications
It is well recognized by the scientific community that microorganisms live without oxygen, for example in deep ocean (Kotlar 2009) and a systematic study of these could lead to other useful applications. One that could be of great benefit to the oil and gas industry would be in preventing the souring of reservoirs. Hydrogen sulphide (H2S) gas poses great safety threat to the drilling and production of oil and gas, in addition to the corrosion challenges. The DNA of the bacteria such as sulfate-recycling bacteria at extraordinary depths and temperatures could be identified and applied to detect, monitor, and modify the biological activity in the environment (de Ferra 2007). The study can be applied to monitoring and controlling the H2S-producing and - consuming bacteria and preventing the souring of the reservoirs. This should help in safe drilling and reduce the corrosion of tubulars.
There are numerous benefits of applying a fundamental understanding of the life cycle of a molecule of oil or gas either in fossil fuel or biomass. This should help the scientific community harness the energy from these sources safely and economically and sustain availability of fuel for decades to come.
1 http://en.wikipedia.org/wiki/Methanogenesis
2 http://en.wikipedia.org/wiki/Thermophilic
References
- Behdad, M.R., Kantzas. A., Pereira-Almao, P., Larter, S. 2010. Catalytic Down-hole Upgrading of Heavy Oil and Oil Sand Bitumens. U.S. Patent Application 60865733.
- De Ferra, F. 2007. Energy: The Next Biotechnology Challenge. JPT 59:1:30-33.
- Ettwig, K. F. et al. Nature 464, 543-548 (2010).
- Galarraga, C., and Pereira-Almao, P. 2010 Hydrocracking of Athabasca Bitumen using Submicronic Multimetallic Catalysts at Near In-Reservoir Conditions. Energy Fuels 24 (4): 2383-2389.
- Grant, B. 2009 Future Oil. The Scientist 23:2:26.
- Jones, D.M., Head, I.M., Gray, N.D., Adams, JU.J., Rowan, A.K., Aitken, C.M., Bennett, H. et al. 2008. Crude-oil biodegradation via methanogenesis in subsurface petroleum reservoirs. Nature 451: 176-181.
- Kotlar, Hans Kristian. 2009. Can Bacteria Save the Oil Industry? The Scientist 23:2:30;
- Krejci-Graf, K. 1932. Rule of Denisty of Oils, AAPG Bull. 16: 1038.
- Mascarelli, A.L. 2009. Gold rush for algae. Nature 461:460-461. DOI:10.1038/461460a. http://www.nature.com/news/2009/090923/full/461460a.html
- Mascarelli, A.L. 2010 Methane-eating microbes make their own oxygen. Nature News, 24 March. DOI:10.1038/news.2010.146. http://www.nature.com/news/2010/100324/full/news.2010.146.html
- Palmer, S.E. 1993. Organic Geochemistry 511-534. ed. Macko, S.A. and Engel, M. H. New York City: Plenum Press.
- Roadifer, R. E. 1987. Exploration for Heavy Crude Oil and Natural Bitumen. ed. Meyer, R.F. Tulsa, Oklahoma: American Association of Petroleum Geologists.
- Sanderson, K. 2007. Oil-eating bacteria make light work of heavy fuel. Nature News.
DOI:10.1038/news.2007.375.
http://www.nature.com/news/2007/071212/full/news.2007.375.html
